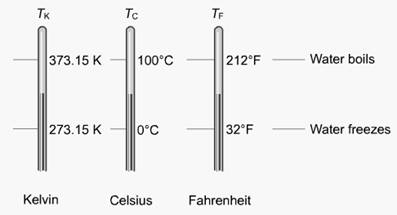
Figure 3.1 temperature scales
|
Back Unit Two
|
Home Cover Page
|
Top Unit Three
|
Next Unit Four
|
Unit Three: Temperature and heat
Starter Activity: Open the discussion by pointing out that everybody is familiar with idea of temperature. Elicit students’ background knowledge about temperature. You hear the word used every day. Then use the following analogies to make learning easy: Mass is a measure of the amount of substance in a system. Velocity is a measure of how fast a system moves .Acceleration is a measure of how fast velocity is changing. But just what is a temperature a measure of ?
Temperature is an indication of the hotness of something. We can tell by touching an object whether it is hot or cold. However, this is a subjective judgment, and is not very reliable. Let us use a simple experiment to demonstrate this. Take three basins of water, each holding the same quantity of liquid, but one containing hot water, a second containing lukewarm water, and the third one cold water. Place one hand in the hot water, and the other in the cold water. Leave them for a few seconds. Now, put both hands together into the basin of lukewarm water, and notice that both hands give different messages. The hand that came from the hot water now feels cold, whereas the hand taken out of the cold water feels warm.
We cannot rely on our skin as an accurate way of measuring temperature. The physiological sensations registered by receptors in our skin depend largely on their immediate past experience, as the experiment illustrates. So, we need thermometers with suitably defined scales for the reliable measurement of temperature.
Rub two sticks together and you will notice that the temperature of each increases. You did work on the sticks and their temperature increased. Doing work is transferring energy. So you transferred energy to the sticks and their temperature increased. This means that an increase in the temperature of a system is an indication of an increase in the internal energy (or thermal energy) of the system.
Another way of increasing the temperature of a pair of sticks is to bring them into contact with something hotter than the sticks are. When you do that, the temperature of the sticks gradually increases—you don’t have to do any work on them. Again, the increase in the temperature of either stick indicates an increase in the internal energy of that stick. Where did that energy come from? It must have come from the hotter object. You may also notice that the hotter object’s temperature decreased when you brought it into contact with the sticks. The decrease in temperature of the hotter object is an indication that the amount of internal energy in the hotter object decreased. You brought the hotter object in contact with the sticks and energy was automatically transferred from the hotter object to the sticks. The energy transfer in this case is referred to as the flow of heat. Heat is energy that is transferred from a hotter object to a cooler object when you bring the two objects in contact with each other. Heat is not something that a system has but rather energy that is transferred or is being transferred. Once it gets to the system to which it is transferred we call it internal energy. The idea is to distinguish between what is being done to a system, “Work is done on the system and/or heat is caused to flow into it”, with how the system changes as a result of what was done to it, “The internal energy of the system increases.”
The fact that an increase in the temperature of an object is an indication of energy transferred to that object might suggest that anytime you transfer energy to an object its temperature increases. But this is not the case. Try putting a hot spoon in a glass of ice water. (Here we consider a case for which there is enough ice so that not all of the ice melts.) The spoon gets as cold as the ice water and some of the ice melts, but the temperature of the ice water remains the same (0 °C).The cooling of the spoon indicates that energy was transferred from it, and since the spoon was in contact with the ice water the energy must have been transferred to the ice water. Indeed the ice does undergo an observable change; some of it melts. The presence of more liquid water and less ice is an indication that there is more energy in the ice water. Again there has been a transfer of energy from the spoon to the ice water. This transfer is an automatic flow of heat that takes place when the two systems are brought into contact with one another. Evidently, heat flow does not always result in a temperature increase.
Concluding Activity: After recapping the core ideas of the lesson, let students attempt to solve the following questions so that you can confirm their level of understandings.
Starter Activity: Initiate the class by pointing out that whenever you measure something, you are really just comparing that something with an arbitrarily-established standard. For instance, when you measure the length of a table with a meter stick, you are comparing the length of the table with the standard meter. In the case of temperature, a standard, now called the “degree Celsius” was established as follows: At 1 atmosphere of pressure, the temperature at which water freezes was defined to be 0 °C and the temperature at which water boils was defined to be 100 °C. Then a substance with a temperature-dependent measurable characteristic, such as the length of a column of liquid mercury, was used to interpolate and extrapolate the temperature range. (Mark the position of the end of the column of mercury on the tube containing that mercury when it is at the temperature of freezing water and again when it is at the temperature of boiling water. Divide the interval between the two marks into a hundred parts. Use the same length of each of those parts to extend the scale in both directions and call it a temperature scale.) Materials whose property significantly and noticeably changes, when heated are referred to have temperature –dependent measurable characteristics. Any property which varies continuously as a substance gets hotter can be used to measure temperature. But to devise an effective method of providing a temperature scale some fixed reference points must be agreed. One such temperature- dependent property is the length of a mercury column contained in a glass envelope.
Main Activity: Suppose you have a mercury thermometer without any degree marks on its stem. To use it to measure a temperature on the Celsius scale, you need to have these two temperatures, called fixed points.
To measure an unknown temperature, we first place the bulb in melting ice, and measure the length of the mercury column. Call this reading L0 .The bulb should then be placed in the steam from boiling water and the length measured again. Call this L100 .We mark these points on the graph, and draw a straight line between them. Once we have marked the fixed points on the stem, we can divide the interval between the two marks into 100 equal lengths. Each length can also be added below the 0 °C mark and above the 100°C mark to measure temperature outside these points. This provides the basis of the ‘mercury –in-glass’ temperature scale.
We can now find an unknown temperature T°C by measuring L and using the graph. Concluding Activity: Liquid-in-glass thermometers have the advantage that they can be used to read the temperature directly. Once the fixed points have been marked on the stem, the space between them can be divided into 100 equal lengths and numbered. We can find an unknown temperature by seeing how long the length L is when the bulb of the thermometer is at the unknown temperature.
Have students practice using thermometers as an assignment. You may want to review this topic by posing some questions .Have some students in one group use a thermometer to measure the temperature outside during different hours, such as the morning, afternoon, and late afternoon. Ask them how the does the temperatures change? Why do they think it changed? Then have another group use thermometers to measure the temperature of a cup of water. Add ice cubes and measure the temperature again
Starter Activity: in this lesson you see thermometers representing three temperature scales. You may be most familiar with the Celsius scale, but the Fahrenheit and Kelvin scales are also used in the sciences. This demands a need to switch from one temperature scale to another. As the thermometers show, temperature values are different on each scale. For example, an average indoor room temperature is 68 degrees on the Fahrenheit scale, which equals 20 degrees Celsius or 293.15 Kelvin.
Main Activity: The temperature at which water freezes is 0° on the Celsius scale. The comparable temperatures are shown on the other scales. There are 100 degrees between the freezing and boiling points of water on the Celsius scale, and 100 kelvins between freezing and boiling on the Kelvin scale. This means the units of these two scales are equal. Since the Celsius and Kelvin scales have the same number of units between the freezing and boiling points of water, it takes just one step to convert between the two systems, as shown in the first conversion formula below.
Since the Celsius and Kelvin systems differ only in their zero points, converting between the two scales is a matter of addition or subtraction. To convert from degrees Celsius to Kelvin, you add 273.15 degrees. To convert from Kelvin to degrees Celsius, you subtract the same value.
TK = TC + 273.15

Figure 3.1 temperature scales
The following equation helps you to convert from degrees Fahrenheit to degrees Celsius.
TC = (5/9) (TF −–32)
TF= (9/5) TC +32
TK = TC + 273.16
Where
TK=Kelvin temperature
TC=Celsius temperature
TF = Fahrenheit temperature
Another important concept shown in the illustration is: absolute zero. At this temperature, molecules (in essence) cease moving. Reaching this temperature is not theoretically possible, but temperatures quite close to this are being achieved. Absolute zero is 0 K, or −273.15°C.
Concluding Activity: Confirm students understanding by providing the class with a quiz and have quick check to see if they have understood the conversions introduced in the lesson. If students have trouble with the conversion, let them reread their textbook, do more practice on conversion problems ; ask their teacher for assistance and guidance , or discuss the material with a fellow student. A simple and yet useful and helpful quiz may look like:
Starter Activity: Initiate the lesson asking students to state some differences between heat and temperature, and then inform them that we will discuss experiments that led us to the abandonment of the caloric theory of heat and its replacement by an energy interpretation of it. Ask them whether they have heard of 18th century scientists thought-that heat was an invisible, fluid substance called caloric or not.
If they are unfamiliar with this idea, you may wish to start this lesson with the introduction of the caloric theory. In the caloric theory heat was thought as a substance with fluid-like properties. If you place a hot object and a cold object together, they evolve toward a common final temperature. Commonsense suggests that “something” flows from the hot object to the cold until equilibrium is achieved. This “heat fluid” was called caloric and every object contained caloric. This theory was proven wrong by Benjamin Thomson. He believed that, if caloric existed, any object being heated or cooled should change mass as the caloric flowed into it or out of it. He designed experiments to test this idea and his findings showed the mass of an object did not change in any way when it was heated. He concluded that caloric did not exist and heat was not a fluid.
Main Activity: Careful experiments conducted by Joule found that we can raise the temperature of a beaker of water by two entirely different means:
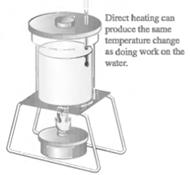
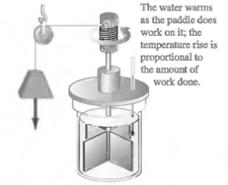
Figure 3.2 Joule’s experiment
The final state of the water is exactly the same in both cases. This implies that heat and work are basically equivalent to each other. In other words, heat is not a substance. Instead heat is the energy that is transferred from a hotter object to a colder object as a consequence of temperature difference between them.
Physicists do not say an object has heat. Heat refers solely to the flow of energy due to temperature differences. Heat transfers thermal energy that is internal to objects, related to the random motion of the atoms making up the objects.
Heat is like work: It changes the energy of an object or system. It does not make sense to say “how much work a system has”, nor does it make sense to say “how much heat the system has”. Just as work is done by a system or on a system, heat as thermal energy can enter a system or leave a system. Having said that heat is measured in joules, we will backtrack a little in order to explain some other commonly used units.
Historically, before the connection between heat and work had been recognized, a unit for measuring heat, the calorie had been defined as :
1 calorie=1 cal=the quantity of heat needed to change the temperature of 1g of water by 1 0C
Once Joule established that heat is energy, it is apparent that the calorie is really a unit of energy. In today’s SI unit the conversion is
1 cal= 4.186J
The calorie you may be familiar with in relation to food in biology lesson; is not the same as the heat calorie, abbreviated Cal with a capital C, is
1 food calorie= 1Cal=1000cal=4186J
The calories you see labeled on the back of food packages − –is actually a kilocalorie Food calories measure how much heat will be released when an object is burned
Concluding Activity: After recapping the core points of the lesson you may want to emphasize that it is especially important not to associate an observed temperature increase with heat. Of course, heating a system is one way of changing the temperature of the system, but Joule showed it is not the only way, since you can also change the system’s temperature by dong work on the system.
Give also emphasis to significance of pointing out that matter does not contain heat. Matter contains molecular kinetic energy and possibly potential energy, not heat. Heat is energy in transit from a body of higher temperature to one of lower temperature. Once transferred, the energy ceases to be heat. As an analogy, work is also energy in transit. A body does not contain work. It does work or has work done on it.)You may wish to test students understanding by posing such questions:
Starter Activity: You can start the lesson asking students “what happens to materials when their temperature changes?’ Pose these questions to elicit students’ experience-Have you ever realized that telephone wires become longer and sag more on a hot summer day than they do on a cold winter day? Have you ever noticed that steel roofs likely produce a cracking sound on hot days? Have you experienced that if one part of a piece of glass is heated or cooled more rapidly than adjacent parts, the resulting expansion or contraction may break the glass, especially if the glass is thick? Then state that in this lesson we see about expansion, its effects and the uses we can make of it.
Main Activity: Precede the lesson stating that when an object absorbs heat energy, various changes in the physical properties of the object may occur. To name some the temperature of the object may rise, accompanied by an expansion or contraction of the object, or the object may liquefy or vaporize during which the temperature remains constant.
Heating things causes' expansion due to the increase in the energy of the particles in the substances .The expansion of solids is small and not usually great enough to be measured with a ruler. Let the teacher demonstrate this simple activity to illustrate expansion of a metal rod.
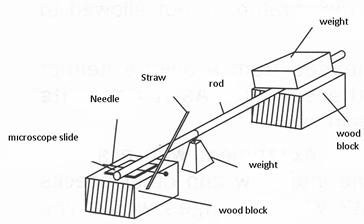
Figure 3.3: expansion of a metal rod
We can show the expansion of metal solids in other ways. Divide the class into smaller groups and assign them to carry out the following hands-on activities:

Figure 3.4: ball and ring
Common misconception: Students usually believe that when the metal surrounding a hole is heated then the hole will get small when it actually should increase in size. This is a good demonstration because it highlights the misconceptions students develop. They like to think that the hole gets smaller. Students find this demonstration very helpful. If a metal plate containing a hole is heated then the whole plate will expand including the hole.
Imagine a circle drawn on the plate with no hole.
Concluding Activity: Recapitulate the key points in concise and clear statements as:
Most solids, like metals expand equally in all directions as they are heated. In the ball and ring experiment, the ball has a bigger radius and thus a bigger volume. Any expansion of the ring would result in the hole having a bigger radius, and thus a bigger area. However guide students by stressing the point that not all solids expand equally in all directions .Finally check students understanding by posing the following questions:
Q1. A steel block is heated so that the length of each side increases 1%. What happens to its mass?
A. It increase by 1 %
B. It increase by 3 %
C. It does not change
Q2. Explain why a thick glass vessel often crack if placed in very hot water.
Starter Activity: Begin this lesson revising what they learnt in the previous lesson about expansion of solids. Remind students that when solids are heated, its particles gain energy and so vibrate with greater speeds; their average distance of separation between atoms increases and this leads to an overall increase in size. We will see the expansion of liquids and gases in this lesson.
Main Activity: Since students are not familiar with the kinetic molecular theory of gases, it is appropriate to support this lesson with experiments to illustrate the basic ideas behind.
Activity on expansion of liquids
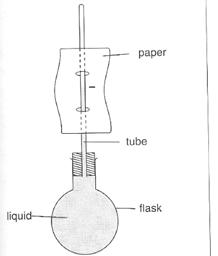
Figure 3.5: expansion of a liquid
The water level rises as you warm the flask, showing that the liquid is expanding. The amount of expansion increases as the water gets hotter. You can take it as a large model of a thermometer, though water would not be used as a thermometric substance in a thermometer.
Keep reminding students that the flask is also expanding as you warm it. It can therefore hold more liquid. Notice that the water level dropped as you started warming the flask. The heat first warms the flask and it expands. Because it can hold more liquid, the liquid level in the tube drops. However, as the heat reaches the water and because the water expands more than the glass, the liquid level soon rises. The actual expansion of the liquid is more than it appears to be.
Activity on expansion of gases-Use the flask used above, but without any water in it. Hold the flask between your hands so that the end of the tube just dips below the surface of water in a beaker, as shown in the figure .The warmth of your hands causes the air to expand, and it bubbles out through the water.
In this experiment, the volume of the gas increases because of the rise in temperature.
You can also try the following simple activity: Take an empty bottle of course; the bottle is not really empty for it is filled with air. Attach a balloon to its neck. Now heat some water in a vessel on a heater or stove. When the water starts boiling, gently place the bottle in boiling water. Look at the balloon. It is inflated. Why is the balloon inflated? The reason is that the air in the balloon expands when its temperature rises. Take the bottle out of the boiling water and allow it to cool. When the air in the bottle is cooled, the balloon is deflated and collapses. The air has contracted on cooling.
Or you can proceed as follows: When the air expands then air cools. There is a demonstration for you all to try right there at your seats. Are you ready? Let everyone have a hand. Put your hands in front of your mouth and with your mouth open the air does not expand. Now bring your mouth down really tight so the air expands as it comes down .Now feel the temperature of the air.
Thermal Expansion of Water
Heating does not always lead to expansion. Water, in the temperature range from 0 0C to 4°C, decreases in volume with increasing temperature. Above 4°C, water expands when heated. Hence water has its greatest density at 4°C. Water also expands when it freezes, which is why ice humps up in the middle of the compartments in an ice cube tray. By contrast, most materials contract when they freeze.
This abnormal behavior of water has an important effect on plant and animal life in lakes. A lake cools from the surface down; above 4°C, the cooled water at the surface flows to the bottom because of its greater density. But when the surface temperature drops below 4°C, the water near the surface is less dense than the warmer water below. Hence the downward flow ceases, and the water near the surface remains colder than that at the bottom. As the surface freezes, the ice floats because it is less dense than water. The water at the bottom remains at 4°C until nearly the entire lake is frozen. If water behaved like most substances, contracting continuously on cooling and freezing, lakes would freeze from the bottom up. Circulation due to density differences would continuously carry warmer water to the surface for efficient cooling, and lakes would freeze solid much more easily. This would destroy all plant and animal life that cannot withstand freezing. If water did not have this special property, the evolution of life would have taken a very different course.
Concluding Activity: After you make a concise, clear concluding remark on the lesson, you can provide them with ample questions and problems to check their understanding.
Starter Activity: Ask the students to cite examples of the usefulness of expansion and contraction. Have them also cite cases in which there are disadvantages. Some examples of the advantages are: thermometers, expansion and contraction of gases in refrigerator, etc. Examples of the latter are: in railroad rails, the laying of sidewalks in bridges, in the construction of buildings
Main Activity: To open a glass container with a very tight lid, you might hold the glass container under hot tap water. When the temperature increases, the metal lid and the glass container both expand.
The expansion of materials due to a temperature change can be useful sometimes, as the jar-opening example demonstrates. Sometimes, it poses challenging engineering problems. For example, when nuclear waste is stored in a rock mass, heat can flow from the waste to the rock, raising the rock’s temperature and causing it to expand and crack. This could allow the dangerous waste to leak out. Knowing the exact rate of expansion can help engineers design storage intended to prevent cracking.
Good engineering practice takes expansion into account. For instance, bridges are built with expansion joints, which have room for expansion as the temperature increases. Gaps must be left between the lengths of railway tracks to allow for the expansion of the steel rails in the hot season.
Metal tyres can be securely fixed round wheels by means of expansion. The tyre is made a little smaller than the wheel which it is to fit. It is then heated so that it expands enough to be slipped onto the wheel when it is hot. As it cools, it contracts and grips the wheel tightly.
Concluding Activity: You can conclude this lesson by emphasizing the point that the expansion of materials is often a nuisance. If a solid or liquid is prevented from expanding, very large forces are exerted. The effects of expansion must be remembered when designs are made. Pose the questions below to confirm student’s conceptual understandings.

Nut is very tight on a screw. Which of the following is most likely to free it?

Starter Activity: Start the lesson providing students with a review questions to enable them remind the key ideas on expansion of solids. Elicit their responses and gear it towards applying the previous knowledge to today’s lesson. Then proceed the lesson pointing out the fact that most objects expand with increased temperature; how much they expand varies by material. In this lesson, we discuss how much they expand in one dimension, along a line. Their expansion is measured as a fraction of their initial length.
Main Activity:
When heated, the rod expands. Here, we concentrate on its increase in length. We call this linear expansion, the change in length measured along one dimension.
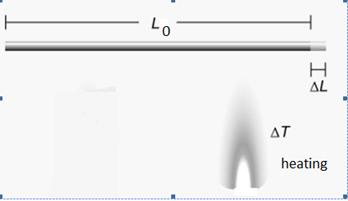
Figure 3.16: Linear expansion of a rod
Consider two rods made of the same material but of different lengths. When the rods expand, the length of the expansion is proportional to the initial length L0 of the rod. These rods are made from the same material, but one is twice as long as the other. Their temperature increases by the same amount, but the longer rod increases in length twice as much. However, the two rods expand the same percentage of their original length, L0 and the ratio of their lengths remains the same.
Imagine also the bimetallic strip made up of two different materials. Their temperatures increase with heat by the same amount, but they expand by different amounts, making the strip curl. A constant called the coefficient of linear expansion specifies how much a given material expands with a change in temperature. The Greek letter α (alpha) represents the coefficient of linear expansion of the material.
The amount that an object’s length changes when it is heated is the product of its initial length, the coefficient of linear expansion for the material of the object and the change in temperature. It is important to remember that this equation calculates the change in length, not the new length.
ΔL = L0αΔT
L0 = original length
α = coefficient of linear expansion
ΔT = change in temperature
Coefficients of linear expansion for materials are based on Celsius or Kelvin temperatures. The change in either scale is the same, since their units are equal. As far as the length goes, any unit of length will work since the equation expresses a proportional change in length.
| Materials | Coefficient of linear expansion at 25 0C |
|---|---|
| Steel | 1.17X 10-5 |
| Iron | 1.18X 10-5 |
| Copper | 1.65X 10-5 |
| Silver | 1.89X10-5 |
| Aluminum | 2.31X 10-5 |
| Magnesium | 2.48X 10-5 |
| Lead | 2.89X 10-5 |
Worked example:
The copper rod is heated from 20°C to 100°C. What will its increase in length be?
You are asked to measure the change in length when the temperature of the rod increases. We start with the equation for linear expansion. The change in temperature is 80 C°.
ΔL = L0αΔT
ΔT = 100°C − 20°C = 80 C°
ΔL = (0.5 m)(1.65×10−5 1/C°)(80 C°)
ΔL = 6.6×10−4 m
In the second step we substitute the values for length, the coefficient of linear expansion for copper, and the change in temperature. The calculation shows that the change in length is less than a millimeter.
Concluding Activity: : Confirm students understanding by providing the class with a quiz and have quick check to see if they have understood the concepts introduced in the lesson. If students have trouble with the expansion equation, let them reread their textbook, prompt them to do more practice on problem solving ; ask their teacher for assistance and guidance on topics of difficulties , or discuss the material with a fellow student. A simple and yet useful and helpful quiz may look like:
Starter Activity: It is appropriate to begin this lesson summing up what students have learnt about linear expansion. You may pose some question just to let them recall the basic concepts of the previous lesson.
Main Activity:
The volume of most materials, including liquids, increases as their temperature increases. For example, the volume of the water in an automobile radiator increases as the engine gets hotter. Most radiators have an overflow tank to capture excess coolant when it expands to a volume greater than the radiator's capacity.
Every substance has a coefficient of volume expansion that determines the relative expansion in volume for that substance for a given temperature increase. The Greek letter (gamma) represents that coefficient. The radiator metal and the coolant have very different coefficients, which is why the fluid overflows the radiator.
Just as with linear thermal expansion, the expansion in volume is proportional to the initial dimensions, in this case the volume of the material.
You see the equation for volume expansion. The change in volume is equal to the initial volume times the coefficient of volume expansion β times the change in temperature, measured in Kelvin or degrees Celsius.
ΔV = V0![]() ΔT
ΔT
Where,
V0 = original volume
![]() = coefficient of volume expansion
= coefficient of volume expansion
ΔT = change in temperature
Coefficient calibrated for K or °C
Notice that for a solid, the coefficient of volume expansion is about three times the coefficient of linear expansion, because the solid expands linearly in three dimensions.
![]() ≈ 3α
≈ 3α
Where
![]() = coefficient of volume expansion
= coefficient of volume expansion
α = coefficient of linear expansion
Worked Example:
The temperature of 2 L of water increases from 10.0° C to 30° C. How much does its volume increase?
You need to use the coefficient of volume expansion of water, which is shown
ΔV = V0![]() ΔT
ΔT
ΔT = 30°C − 10°C = 20 C°
ΔV = (2 L) (2.07×10−4 1/C°)(20 C°)
ΔV = 0.0083 L
In the first step, we write the equation for volume expansion. We calculate the change in temperature, and then enter the values for initial volume, coefficient of volume expansion, and the change in temperature. The volume increases by 0.0083 liters, which equals about half a tablespoon.
Concluding Activity:
Check students’ understanding by providing the class with numerical problems, so that they can solve it in group discussion; and have quick check to see if they have understood the concepts introduced in the lesson. If students have trouble with the expansion equation, prompt to do more practice on solving problems, let them reread their textbook, ask their teacher for help, or discuss the material with a fellow student.
Starter Activity: Initiate the classes by asking some review questions on the relationship between heat and work which lead to discussion of measuring heat. Direct their answers to the point that, to be scientific in the study of heat, we must be able to measure the quantity of heat given off or absorbed by a given mass of a substance when it is cooled or heated through a given change in temperature and that some standard must be used for this measurement. Or you may begin the lesson providing common experiences in daily life as:
If you touch a hot stove, energy enters your hand because the stove is warmer than your hand. When you touch a piece of ice, on the other hand, energy passes out of your hand and into the colder ice. The direction of spontaneous energy transfer is always from a warmer thing to a neighboring cooler thing. The energy transferred from one thing to another because of a temperature difference between the things is called heat.
Main Activity:
How much heat flows depends not only on the temperature difference between substances but on the amount of material as well. For example, a barrelful of hot water will transfer more heat to a cooler substance than a cupful of water at the same temperature. This is because; there is more internal energy in the larger amount of water.
Different substances have different capacities for storing internal energy. In other words, different materials absorb or release energy in different ways. For example, if you wait a short while before eating a piece of hot roast beef and pieces of mashed potatoes, both initially at the same temperature, you’ll find that the meat has cooled off more than the potatoes. Similarly, If we heat a beaker with water on a stove, we might find that it requires 15 minutes to raise it from room temperature to its boiling temperature. But if we put an equal mass of iron on the same flame, we would find that it would rise through the same temperature range in only about 2 minutes. This implies different materials require different amounts of heat to raise the temperature of a given mass of the material by a specified number of degrees.
Specific heat is a property of a material. It is a constant that tells how much the temperature of a mass of the material changes when a particular amount of heat is transferred.
Each matter has its own characteristics to absorb heat. It is the distinguishing property of matters.
A material’s specific heat is determined by how much heat is required to increase the temperature of one kilogram of the material by one Kelvin. A material with a greater specific heat requires more heat per kilogram to increase its temperature a given amount than one with a lesser specific heat. In spite of its name, specific heat is not an amount of heat, but a constant relating heat, mass, and temperature change.
Q = cmΔT
Where
Q = heat
c = specific heat (J/kg·K)
m = mass
ΔT = temperature change in C° or K
This is an equation that uses specific heat. The heat transferred to or from an object equals the product of its material’s specific heat, the mass of the object and the change in temperature. Specific heat is measured in joules per kilogram Kelvin.
We can think of specific heat as thermal inertia. Recall that inertia is a term used in mechanics to signify the resistance of an object to a change in its state of motion. Specific heat is like a thermal inertia, since it signifies the resistance of a substance to a change in temperature.
| Substances | Specific heat(J/Kg.K) |
|---|---|
| Lead | 129 |
| Silver | 235 |
| Copper | 385 |
| Iron | 449 |
| Carbon | 709 |
| Aluminum | 897 |
Explain the point that the specific heat of water is considerably larger than that of the other substances. Its high specific heat means that for a given quantity of heat energy absorbed by water, its rise in temperature will be comparatively small. Thus water, because of its large specific heat, is used in many types of heat exchanger. Large bodies of water such as lakes, seas or oceans tend to moderate variations of temperature nearby since they can absorb or release large amounts of thermal energy while undergoing only very small changes in temperature. The specific heat of water is five times that of the earth or sand, and the water takes longer to heat up than the land. Hence we can say that the high specific heat of water affects the climate near the sea and large lakes.
Other familiar example is the use of water in the cooling system of a car. It removes much of the heat energy that would otherwise cause damage to the engine.
Because so much of our body is composed of water our muscles do not overheat. If we engage in exercise or strenuous physical work, the body temperature does not rise excessively because of the body’s high specific heat.
Concluding Activity: Confirm students understanding by providing the class with a quiz and have quick check to see if they have understood the concepts introduced in the lesson. If students have trouble with the quiz, let them re-read their textbook, ask their teacher for help, or discuss the material with a fellow student. A simple and yet useful and helpful quiz may look like:
Starter Activity: Initiate this lesson by pointing out that the matter in our environment exists in three common phases (or states). Ice, for example, is the solid phase of H2O. Add energy, and you add motion to the rigid molecular structure, which breaks down to form H2O in the liquid phase, water. Add more energy, and the liquid changes to the gaseous phase.
Then in this lesson we see why a kettle of water can be boiled for hours without changing its temperature and that the sun in summer can shine for hours on a large ice without changing either the temperature of the ice or the temperature of the water that drips from it.
Main Activity: In everyday language, the three phases of matter are called ice, water, and steam. That is, the term water implies the liquid phase, ice the solid phase and steam the gas phase.
When ice which is at a temperature below 0 0C is allowed to warm up slowly, its temperature rises to 0 0C and then stays at that value while the ice melts. When all the ice has melted, the temperature will rise above 0 0C.The temperature at which the solid-to -liquid change occurs is called the melting point. That is, it takes energy to liberate the water molecules from the crystal structure of the ice and allow them to move freely at the same temperature through the liquid. This occurs as the ice melts, changing phase from a solid to a liquid. Phase changes between solid, liquid and gas do not change an object’s temperature, but they do require heat transfer. Phase change is a transformation between solid and liquid, liquid and gas, or solid and gas. The phase of matter depends on its temperature and the pressure that is exerted on it. Changes of phase almost always require a transfer of energy.
If we cool a pure liquid, it changes to a solid at the same temperature as its melting point. This is called the freezing point. Impure solids and mixture melt over a range of temperatures; they do not have a definite melting point .A very good example of this behavior is wax.
When energy is supplied to water, its temperature rises and, if the rate of supply of energy large enough, the water boils. The temperature remains constant as the water boils. This temperature at which liquids changes to gas by boiling is referred to as boiling point.
Elaborate also that the boiling point depends on the atmospheric pressure. When water boils, bubbles of steam are formed in the liquid and rise to surface. The pressure of the vapor in the bubble has to be a little greater than the external pressure for the bubble to exist. At normal atmospheric pressure, this happens at 1000C. Bubbles in the liquid can form only when the pressure of the vapor within the bubbles is great enough to resist the pressure of the surrounding liquid. Unless the vapor pressure is great enough, the surrounding pressure will collapse any bubbles that may form. At temperatures below the boiling point, the vapor pressure in bubbles is not great enough, so bubbles do not form until the boiling point is reached If the pressure is lower than the normal, bubbles can form at some temperature below 1000C, so that the water boils at a lower temperature. Water boils at temperatures well below 1000C at the top of high mountains, and cooking food becomes a problem. If the temperature of the boiling water is too low, food will not cook at all. It is important to note that it is the high temperature of the water that cooks the food, not the boiling process itself.
As water is changed into steam, the energy that we supply does not cause a rise of temperature. It supplies the energy necessary to enable molecules to escape from the liquid, and do work against forces biding them together as a liquid. This latent heat is known as the latent heat of vaporization.
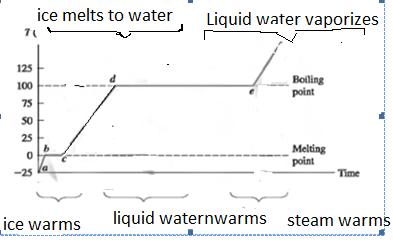
Figure 3.17: phase changes
To change a mass m of a material to a different phase at the same temperature (such as liquid to solid or liquid to vapour) requires the addition or subtraction of a quantity of heat. The amount of heat is equal to the product of m and L, the heat of fusion, vaporization, or sublimation.
Q= +mL
Where
Q=quantity of heat absorbed or released
m= mass of the body
L= latent of heat
Latent heat is energy used for loosening or breaking bonds between molecules and not for raising temperature. There are two forms, viz. latent heat of fusion, LF , and latent heat of vaporization, LV.
Common misconception: There is a tendency to believe that any time heat is flowing into ice, the ice is melting. NOT SO. When heat is flowing into ice, the ice will be melting only if the ice is already at the melting temperature. When heat is flowing into the ice that is below the melting temperature, the temperature of the ice is increasing.
Concluding Activity: After you make a short, concise , summary or concluding remarks which highlight the underlying concepts of the lesson make sure students’ conceptual understanding by providing such kind of concept test questions ;
Confirm that their answers agrees with the following explanations:
Starter Activity: Start this lesson asking students’ experience of boiling water. .You may wish to ask them questions such as: Have you ever observed that when water is coming to boil it starts to murmur? Why? Elicit their reply and continue with your detailed explanation.
Main Activity: Let us heat a glass vessel with cold water on a burner and watch the process. The bottom and the walls of the glass will soon be covered with small bubbles. These bubbles contain air and water vapour. Observing a bubble at a constant temperature implies that it retains its volume. This means that the internal pressure in the bubble is balanced by the external pressure on its surface. Since the bubble contains air whose amount should be considered, this equilibrium is stable.
When some bubbles rise to the surface, their volume is reduced .Why does this occur? Notice that these bubbles contain water vapour and a small amount of air. When a bubble arrives colder upper layer of water, a considerable amount of water vapour condenses, and the bubble contracts. This alternating increase and decrease in the volume of bubbles is accompanied by a noise: water coming to the boil starts to ‘murmur’. Ultimately, the entire mass of water is heated. At this stage, the volume of rising bubble does not decrease any longer, and the bubbles burst at the surface ejecting steam to the surrounding space. The murmuring is now changes to a loud bubbling sound, and the water is said to boil.
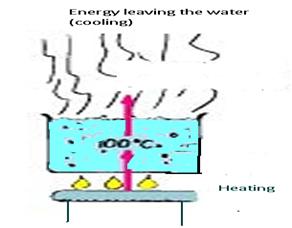
Figure3.18: boiling is a cooling process
Under the right conditions, evaporation can take place beneath the surface of a liquid, forming bubbles of vapor that are buoyed to the surface where they escape. This change of phase throughout a liquid rather than only at the surface is called boiling.
Evaporation is a cooling process. So is boiling. At first reflection, this may seem surprising perhaps because we usually associate boiling with heating. But heating water is one thing; boiling is another. When 100°C water at atmospheric pressure is boiling, its temperature remains constant. That means it cools as fast as it warms. If cooling didn't take place, continued input of energy to a pot of boiling water would result in a continued increase in temperature. The reason a pressure cooker reaches higher temperatures is because it prevents normal boiling, which in effect prevents cooling.
Concluding activity: After you recapitulate the underlying concepts of the lesson, provide these conceptual questions to confirm whether they meet the lesson objectives or not. Use the feedback for the better improvement of the lesson.
Starter Activity: Commence this lesson by mentioning the point that when the temperature drops outside, you put on your winter clothes. By wearing heavier, thicker clothing you are preventing your body from loosing too much heat. Keeping thins cool by keeping heat out is often just as important as keeping heat in. You can cite example as: when you are out in the sun, you may wear a hat to keep your head cool. Or you prefer to use a heavy mug to keep your hot drink warm longer than a thin –walled china cup does. In this lesson we see the mechanisms of heat transfer and how to control of heat transfer.
Main Activity: Proceed with your exposition of heat transfer as –Imagine grabbing the steel handle bars of your bicycle after it has been sitting out in the sun on a hot summer day. Now imagine picking up a piece of wood that is about the same size as the handle bars and that has also been sitting out in the sun. Which do you think would feel hotter? Ask students to suggest reasons. The handle bars are hot because of the metals ability to transfer the heat to all parts of it.
Or you may elaborate it as-for example, if you hold one end of a long metal bar and insert the other end into a flame; you will find that the temperature of the metal in your hand soon increases. The energy reaches your hand by means of conduction. You can understand the process of conduction by examining what is happening to the microscopic particles in the metal. Initially, before the rod is inserted into the flame, the microscopic particles are vibrating about their equilibrium position. As the flame heats the rod, the particles near the flame begin to vibrate with greater and greater amplitudes. These particles in turn collide with their neighbours and transfer some of their energy in the collisions. Slowly, the amplitudes of vibrations of metal atoms and electrons farther and farther from the flame increase until, eventually, those in the metal near your hand are affected. This vibration is detected by an increase in the temperature of the metal and of your potentially burned hand. The transfer of heat by the collision of particles in the solid is called conduction. Conduction is the direct flow of thermal energy without a net motion of the materials involved.
How well a solid object conducts heat depends on the bonding within its atomic or molecular structure. Solids made of atoms that have one or more outer electrons conduct heat (and electricity) well. Metals have loosely bound outer electrons, which are free to carry energy by collisions throughout the metal. They are excellent conductors of heat and electricity for this reason. Silver is the best, copper is next, and, among the common metals, aluminum and then iron are next in order. Wool, wood, straw, paper, cork, and Styrofoam, on the other hand, are poor conductors of heat. The outer electrons in the atoms of these materials are tightly attached. Poor conductors are called insulators.
A cooking pan is a good example of an application of conduction. The pan itself is usually made of metal (a good conductor) so that the heat is rapidly applied to all contents to cook them evenly.However, we do not want the handle to be too hot. The handle should be made of a poor conductor, such as wood or plastic.
Let students in groups conduct activity on rate of heat conduction. Four students, each holding a rod of a different substance in a flame, will demonstrate the difference in conductivity of heat by their object from the flame. Use about the same sized rods of iron, aluminum, glass, and copper Concluding Activity: Check students’ understanding of heat transfer by providing concept tests which will lead to a better understanding of the concept. A room has one wall made of concrete, one wall made of copper, and one wall made of steel. All of the walls are the same size and at the same temperature of 20°C. Which wall feels coldest to the touch? (i) the concrete wall; (ii) the copper wall; (ill) the steel wall; (iv) all three walls feel equally cold to the touch.
Check that their line of reasoning agrees with the following: Answer: (ii) when you touch one of the walls, heat flows from your hand to the lower-temperature wall. The more rapidly heat flows from your hand, the colder you will feel. Recall that the rate of heat flow is proportional to the thermal conductivity. Note also that, copper has a much higher thermal conductivity (385.0W/m'K) than steel (50.2W/m'K) or concrete (0.8 W/m. K), and so the copper wall feels the coldest to the touch.
Starter Activity: Commence this lesson by reviewing the previous lesson as: You have learned how heat transfer takes place in solids by conduction .But not all matter is solid. Liquid and gases are phases of matter in which the particles flow from place to place. They are called fluids, and they are poor heat conductors. Pose this question to the class - If liquids and gases do not conduct heat as well as solids do, how does heat transfer take place in fluids? Let students write their predictions on their notebook.
Main Activity: You may wish to proceed with your exposition as follows –Imagine about soup. How does the soup in a pot on the stove become hot enough to boil? To warm the soup more quickly, you can stir it with a spoon or a stick. As you stir a pot of soup on the stove with a spoon, you can feel the spoon become hot; heat being transferred by conduction along the spoon. As soup is heating, some parts of it, near the heat source, will be hotter than other parts. By stirring, you are mixing higher -temperature soup with lower -temperature soup. This also occurs naturally; because particles in liquids and gases can flow and move past each other. This movement is called convection. Convection is the transfer of heat by mass motion of a fluid from one region of space to another. It isheat transfer through a gas or liquid caused by movement of the fluid. Convection takes place only in fluids. It cannot take place in solids as the atoms/molecules are fixed in their positions relative to each other.
You can also conduct the following hands-on activities to investigate how heat transfer by convection occurs in liquids and gases.
Convection in liquids:
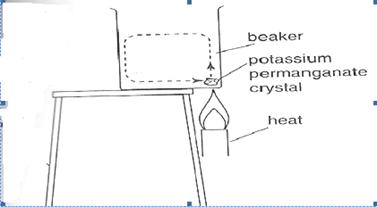
Figure 3.19: convection in liquids
Convection in gases:
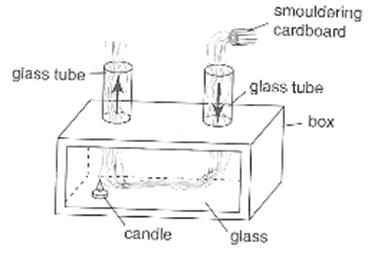
Figure 3.20: convection box
Concluding Activity: You can recap this lesson by stressing on convection currents and noting the point that convection in liquids and in gases is very similar.
Starter Activity: You can begin this lesson by reviewing the previous lesson as-you have learned that heat transfer by conduction and convection depends on the motion of particles .In conduction the particles, move back and forth, colliding with each other. These collisions transfer energy from one particle to the next, but the particles themselves do not move very far. In convection, the particles move through a fluid, transferring energy as they collide with other particles. You know that the sun is the main source of Earth’s energy. The space between the Earth and the sun contains almost no particles, so heat transfer by either of these two methods, conduction and convection, is impossible. Then pose the question ‘how is the sun’s energy transferred to the Earth?’
Everyone has felt the warmth of the sun's radiation and the intense heat from a glowing coal in a fireplace. Most of the heat from these very hot bodies reaches you not by conduction or convection in the intervening air but by radiation.
Main Activity: The third means of energy transfer that we shall discuss is radiation. All objects radiate energy continuously in the form of electrometric waves (see the next chapter) produced by the thermal vibrations of the molecules. Mention that if you place your hand near a red-hot heating element and feel your hand warm up, you are experiencing thermal radiation: the transfer of energy by electromagnetic waves. You correctly think of objects like the heating element as radiating heat; in fact, every object with a temperature above absolute zero radiates energy. You are familiar with electromagnetic radiation in the form of orange glow from an electric stove burner, or the coil of a toaster. Radiation does not require particles to transfer energy. Energy transferred by radiation is referred to as radiant heat.
You can allow students in group try out this simple activity which enables them to discover some of the properties of radiant heat. Let them hold their hands briefly near the incandescent light source and record what they feel. Then let them hold their hands near the fluorescent light source and record their feeling. Finally pose this question to lead them to group discussion: which light source produce more radiant heat?
You can also give the class a project work to discover the emitting radiation as:
Precede the discussion to draw the conclusion that a dull black surface is gives out or emits much more radiation than a polished surface at the same temperature. The best emitter is a dull black surface; silvery polished surface is the worst.
Let students also try the following hands-on activity to investigate absorbing radiation-
Provide students guidance and support to arrive at a concluding remark that the dull black surface takes in or absorb energy much more quickly than the silvery surface. All surfaces absorb energy but the best absorber of radiation is a dull black surface; it absorbs all of the radiation falling on it if it is a truly black .The worst is a silvery polished or white surface, which is a good reflector of radiation. Stress the point that good emitters are good absorbers, and poor emitters’ are poor absorbers.
Concluding Activity: After recapping the core points of the lesson confirm students’ conceptual understanding posing the following conceptual questions.
Check that their answer agree with the following short explanations or not.
Note for the teacher: Heat transfer by radiation is important in some practical applications, for example in a thermos flask the flask is a double-walled glass vessel which has been evacuated and sealed. The air is pumped out of the spaces between the walls; this eliminates nearly all heat transfer by conduction and convection. The silver coating on the waIls reflects most of the radiation from the contents back into the container, and the waIl itself is a very poor emitter. Thus a vacuum bottle can keep coffee or tea hot for several hours. The thermos flask is often used to keep liquids very cold; store very cold liquefied gases.