Unit Five: Physical States of Matter
Lesson 3: Phase change and Energy Change in Solids
Competencies
- Demonstrate an experiment to show the phase changes from ice to liquid water and then to water vapour
- Explain the phase changes
- Describe the processes of phase changes
- Explain the energy changes in phase changes
Materials
Pyrex beaker of 200ml, thermometer, tripod with wire gauze, stirrer, stand with clap, crashed ice (voluntary students may be asked to bring from nearby village),
Note for the teacher
The concept students understand in this activity is the latent heat of vaporization and latent heat of fusion. These concepts can only be clear when practically performed. Students should be able to explain why temperature is not raised at melting points and boiling points. To understand this, careful performing, recording and analysing of the data is required. Therefore, students should be assisted to conduct and observe the activity.
Starter activity
You may start the lesson by revising changes of state. This can be done by matching terms used for the processes of changes with the changes.
Procedure
- Write each term on each of the same coloured card and the descriptions on the other with different kind of colour.
- Provide the two series of cards for each group of students.
- Let the students match card of the correct process or change to the descriptions.
- The terms can be
- Vaporization
- Condensation
- Deposition
- Crystallization
- Sublimation written on one kind of card
- The processes are:
- Change of gas to liquid
- Change of solid to liquid
- Change of liquid to solid
- Change of gas to solid
- Change of solid to gas written on the other.
Check whether the students successfully matched or not and help them when there is a problem.
Evaluation
Students may be asked questions like:
- Classify the following processes as endothermic and exothermic: Vaporization, Sublimation, Condensation, Deposition and Crystallization
- Given the following energy changes, pair the ones which are the reverse of one another. Heat of crystallization, heat of fusion, heat of condensation, heat of deposition, heat of vaporization and heat of sublimation
Main activity (26 minutes)
Let the students do the activity in groups if there are available materials. If not you can demonstrate the experiment by involving the students.
Procedure
- Add the crashed ice into the beaker
- Put the beaker on the tripod with wire gauze
- Insert a thermometer into the ice and fix it with a clamp of the stand so that it will not touch the bottom of the beaker.
- Heat the ice in the beaker, measure the temperature and record the temperature every 30seconds
- Continue your observation and recording until the water boils
- You may record your data in table as follows
Time (s) |
0 |
30 |
60 |
90 |
120 |
150 |
180 |
210 |
240 |
270 |
300 |
Temperature (0C) |
|
|
|
|
|
|
|
|
|
|
|
- Draw a graph by using time on the x-axis and temperature on the y-axis.
Analysis
- What was the phase of the content of the beaker at the beginning?
- To what temperature was the thermometer reading raised at the beginning?
- What was the phase during the first constant temperature?
- What has happened at this temperature?
- At what thermometer reading was the second raise in temperature observed? To what temperature was it raised?
Conclusion
- What do you call the first temperature at which the thermometer reading was constant?
- Why doesn’t the temperature raised while the heating process was continued?
- What was the purpose of the heat consumption during the constant temperature?
- Why was the temperature raised for the second time?
- Why the temperature was remained constant for the second time? What do you call this temperature?
- What do the compartments of the curve you drawn show?
Generalization
- What is the melting point of ice at the particular conditions of your environment? How do you define melting point?
- Why does temperature remains constant at melting point?
- Why does temperature remains constant at boiling point? What is boiling point?
- Are fusion and vaporization endothermic or exothermic processes?
- Is it possible to reverse these processes?
- What should we do to reverse the processes?
Let the group representatives present their observation, conclusion and generalization. The other groups can discuss the findings in comparison to their works. The following main points should be checked whether the students grasped or not?
- Ice melts at about 00C (may be there is slight variation due to altitude)
- Temperature remains constant at melting point until all the ice melts. The heat is consumed to break the intermolecular forces between water molecules instead of raising the temperature of the system.
- Below the melting temperature the content of the beaker was solid, at a melting point the solid and the liquid coexist, between m.p and b.p liquid exists and above b.p liquid and vapour exist
- Temperature remains constant at boiling point until all the liquid vaporizes. The heat is consumed to break apart the force between liquid molecules.
- The heats consumed without raise in temperature are called latent heats. The first one is called latent heat of fusion while the second is latent heat of vaporization.
Evaluation
Students should be able to answer the following questions.
- Why are melting and boiling endothermic processes?
- What are latent heats of fusion and latent heat of vaporization?
- How do you explain melting and boiling in terms of the relationship between particles of substances?
Make sure that students can successfully explain that during melting and boiling intermolecular forces break up; as the result heat energy is required.
Latent heats are heats that are consumed without raise in temperature.
Particles are strongly attracting and are close to each other in solid crystals. Melting is then breaking this strong attraction force so that the particles can free to flow. In boiling particles of liquid which are still under attraction of each other are set completely free from each other.
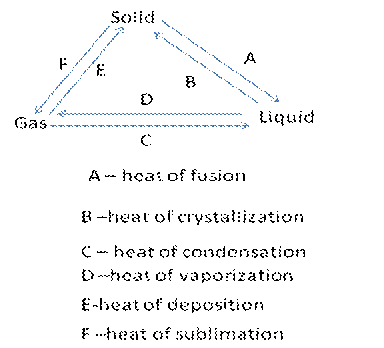
Concluding activity
You can give the following activity to the students
Procedure
- Draw a triangle using the words solid, liquid and gas at each corner of the triangle
- Write double arrows with opposite direction between the vertices
- Write the energy involved to change one phase to the other