|
Back Unit 5.1
|
Home Cover Page
|
Top Unit 5.2
|
Next Unit 5.3
|
Unit Five: Physical States of Matter
Materials
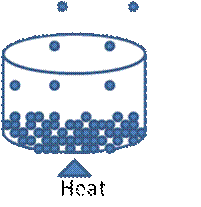
Diagrammatic representation of vaporization
Note for the teacher
The lesson of vapour pressure can be contextualized because students have experiences in their day-to-day activities. The most important point is understanding what happens at particle level during vaporization and why do different liquids have different vapour pressures? Students should also understand the concepts such as volatility and viscosity since they encounter them in their lives frequently. To depict the concept of vapour pressure it is advisable to conduct the experiment on the student textbook page 193.
The lesson can be started by inviting students to discuss the process of cooking and boiling in the kitchen at home.
Let the students discuss round their desks the following points;
Give them some time and ask them to explain their understandings.
Although the concept seems simple, students may come up with different views regarding the phenomena. Some of them may say ‘the liquid is splashed up to the cover”, the others may think simply the surroundings of a liquid is usually wet like river sides. Some the students may guess that liquid vaporizes and condenses on the cover.
Regarding the removal of the cover of the cooking utensil, they may also think that the liquid it self pushes the cover. Some of them may unable to imagine that vapour pressure has such energy. They should also be able to draw figure like the following one.

Evaluation
What makes the cover of the cooking utensil wet during cooking?
What pushes the cover off the cooking utensil?
Now you may define vapour pressure as the partial pressure developed above a liquid. Let the students investigate the factors that affect vapour pressure of a liquid by doing the following activities
Organize the students in to groups and give them to the following activities to discuss and come up with kinetic theory of matter explanations.
Activity 1
If you keep equal amount of water in equal sized open beakers, the first being under temperature of 200C and second under temperature of 450C , the content of the second water reduces significantly than the first one.
Activity 2
If you keep equal amounts of benzene and water in equal sized open beakers under the same temperature, the volume of the benzene reduces significantly in the same time.
The group representatives should present their observations to the class. The class may ask questions and discussion should be extended among students.
You should assist the students in their discussion to associate the concepts to the kinetic theory of matter and the intermolecular forces of attraction.
Students should come to the understandings like:
Evaluation
Students can be asked question like:
Concluding activities
Let the students discuss and classify the following substances as volatile and non-volatile. The substances are: ammonia, water, benzene, ethanol, perfume, sulphuric acid, and edible oil.
Check whether the students can perform the classification as
| Volatile | Non-volatile |
Ammonia |
Water |
Benzene |
Sulphuric acid |
Ethanol |
Edible oil |
Perfume |
|