Substance
Ice →
water →
water vapor
Physical state
Solid
Liquid
Gas
Description 1
![]()
![]()
![]()
![]()
![]()
![]()
Description 2
![]()
![]()
![]()
![]()
![]()
![]()
Description 3
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
|
Back Unit 3.3
|
Home Cover Page
|
Top Unit 3.4
|
Next Unit 4.1
|
Unit Three: Chemical Bonding and Intermolecular Forces
Material
Charts with diagrams of different types of molecules with different inter molecular forces
Note for the teacher
Students may have difficulties in understanding intermolecular forces. For example they may think that the whole system of solid or liquid covalent compounds is formed by covalent bonding. More likely that is the reason why they believe covalent bonds are broken when a substance changes state. They may think that when water boils, the vapor contains hydrogen and oxygen atoms. Indeed, they may attribute decreased melting and boiling points of covalent substances to weak covalent bonds.
This lesson may be started by asking students background knowledge on their preconception about inter molecular forces from their daily life experiences.
Let them discuss across their tables the following:
How do you explain the changes of state in water? Consider the angular shape as water molecule; the larger sphere as oxygen and the smaller as hydrogen. Choose the correct graphic description of the changes of state among the alternatives 1, 2 and 3.
If students assume presence of covalent bond breakage during a change of state they prefer graphic description 1. On the other hand if they have imagined that there is no change at particle level during change of state, they choose description 2.
However, students should come to the conclusion that there are attractive forces between the molecules of substances. This forces breaks up during a change of state from solid to liquid and then to gas. They already know from their lower grades that physical changes do not involve change of composition. However, most students fail to relive and link concepts.
You can now explain intermolecular forces are attractive forces which hold the molecules together. On the other hand intra-molecular forces are chemical bonds like ionic and covalent bonds that hold the atoms together to form a molecule or a compound.
Substance |
Ice → |
water → |
water vapor |
Physical state |
Solid |
Liquid |
Gas |
Description 1 |
|
|
|
Description 2 |
|
|
|
Description 3 |
|
|
|
You may ask the students the following questions
Let the students perform the following activity. Given the molecules: HCl, H2, O2, HBr, H2O, NH3, Ne, HI and He; let them
Figures
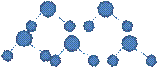
1. 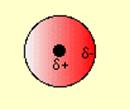 2.
2. 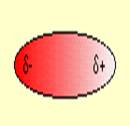
Let the students discuss in groups across their table and try to associate the concepts of polarity and non-polarity to the molecules and find out their representation from the given figures.
Some students can successfully match the polar molecules to the polar figures and tell that the positive and negative terminals attract each other. But still some of them may unable to link the concepts. Let the able students explain their observations and then you can give summary afterwards that polar molecules have dipoles due to the difference in the electro negativity of their constituent elements. The molecules align themselves with the positive terminal close to the negative terminal. So, due to the electrostatic attraction between the partial positive and partial negative charges, the molecules develop intermolecular attractive force which is called dipole-dipole attraction force.
The second groups of molecules can be explained by analogy as follows. When you take a positive terminal of a magnet to an iron nail, the nail is attracted to the magnet. This is because the positive end of the magnet pulls the electrons in the nail forward and causes the other end to be positive. If you bring another nail closer to first nail, it is attracted in the same way as the magnet did to the first nail. So, the magnet developed opposite charges into the first nail and the first nail induced the charge into the second nail.
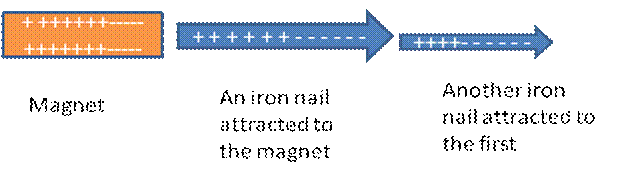
Molecules can induce charges into one another in the same way. The electrons in the molecules are continuously vibrating. This instantaneous motion of electrons shifts them to one end at one time and to the other end at another time. When one end becomes negative due to the accumulation of electrons, the other end becomes positive. The charged molecule then develops induced dipole in to the neighbouring molecules. As the result the molecules attract each other. Such force is called instantaneous dipole- instantaneous dipole attraction force. It is also called London force after its discoverer, the German-American physicist, Fritz London.
You may ask the students the following questions
Let the students discuss on the properties of the substances which are influenced by the intermolecular forces. Challenge them by giving clue to explain properties like melting point, boiling point that depend on intermolecular forces.
Students should also know that the two types of intermolecular forces they studied above are collectively called Van de Waal’s forces. London force is the weakest of all the intermolecular forces and exists in all kinds of molecules.