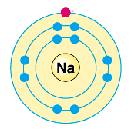
Na
→
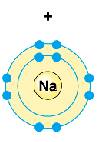
Na+
+
e_
1s22s22p63s1
1s22s22p6
2, 8, 1
2, 8
Na•
Na+
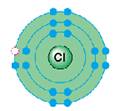
Cl
+
e-
→
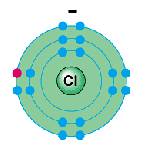
Cl-
1s22s22p63s23p5
1s22s22p63s23p6
2, 8, 7
2, 8, 8
![]()
![]()
|
Back Unit 3.1
|
Home Cover Page
|
Top Unit 3.2
|
Next Unit 3.3
|
Unit Three: Chemical Bonding and Intermolecular Forces
Materials
Note for the teacher;
This lesson is a lesson that should be delivered next to a lesson on ion formation in the sub-topic “formation of ionic compounds”; i.e the second lesson of the three lessons on ionic bond formation.
Start this lesson, by explaining that metal atoms give electrons and form cations, whereas non-metals lose electrons and form anions. These two attract each other by the electrostatic attraction force between oppositely charged ions to form ionic bond. Therefore, ionic compounds are made up of non directional bond in which all the oppositely charged ions attract each other in all directions and like charged ions repel each other. However, the common misconception here is that students believe that ionic compounds like NaCl are diatomic molecule. They fail to understand that ionic compounds are aggregates of ions. So, this lesson is designed to further clarify ionic bonding by using Styrofoam ionic models. The use of the model is not to teach the three dimensional crystal structure which is beyond the scope of the lesson but to describe that an ionic bond is non directional and the ionic compounds do not have molecular particles.
The lesson may be started by revising the formation of ions. You may write the symbols of some metals like Na, K, Mg and Al and that of the non-metals like Cl, F, O, and N; ask some students to write ionization reaction (i.e. lose of electron for the metal atoms to form cation and gain of electron for the non-metals to form anion) , some of them to write electron configuration of the atoms and their ions, the rest to write Bohr’s models for the atoms and ions and finally Lewis/dot formula of the atoms and the ions. In this way you can involve many students in the revision activities.
It is also important to remind students about the interactions between opposite and like charges from their physics lessons.
Make sure that students successfully accomplish the above tasks as follows:
Na |
→ |
Na+ |
+ |
e_ |
1s22s22p63s1 |
|
1s22s22p6 |
|
|
2, 8, 1 |
|
2, 8 |
|
|
Na• |
|
Na+ |
|
|
|
|
|
|
|
Cl |
+ |
e- |
→ |
Cl- |
1s22s22p63s23p5 |
|
|
|
1s22s22p63s23p6 |
2, 8, 7 |
|
|
|
2, 8, 8 |
|
|
|
|
|
|
|
|
|
|
Students may be asked the following questions
Students may be asked to predict what happens when sodium and chlorine atoms are brought together. Let them discuss around their table, write down their impression and report to the class.
Some students may come up with correct answers from their previous knowledge of octet number and electron gain and lose. The others may be unable to bridge the last and the present concepts.
Using the more able students’ ideas you can assist the class to associate the concepts of formation of cations and anions and electrostatic attraction between oppositely charged particles.
Let students exercise writing formation of cations and anions and their attraction to form ionic bond using Bohr models, Lewis symbols and chemical reactions as shown in the starter activity table of this lesson.
Now ask students which structure they would choose for sodium chloride formed when atoms of sodium and chlorine combine.
Consider the smaller red sphere as sodium cation and the green larger one as chloride ion.
Which of the following is the most likely structure for sodium chloride?
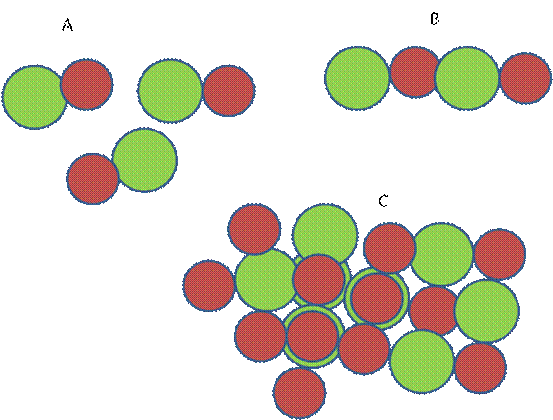
D
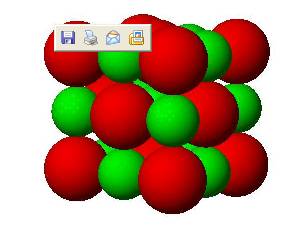
Students may tend to choose “A” as a model for the structure of sodium chloride because probably they think that it is made up of separate molecules. However, it should be clear that each positive sphere or cation, attracts the opposite charged anions in all direction and the anions also attract the cations in all directions. As a result the number of anion surrounding the cation depends on the relative size of the ions. As there are forces of attractions between opposite charges and forces of repulsion between like charges, oppositely charged ions are placed as close as possible and the like charged ions as far apart as possible. In the case of sodium chloride each sodium ion is surrounded by six chloride ions and each chloride ion in tern, is surrounded by six sodium ions Such repetitive arrangement of the ions form a giant structure.
Now instruct the students to construct a three dimensional structure for sodium chloride using the atomic models or Styrofoam balls and a glue or gum.
Group Period |
IA |
IIA |
IIIA |
IVA |
VA |
VIA |
VIIA |
1 |
|
|
|
|
|
|
|
2 |
A |
|
|
|
G |
D |
|
3 |
|
B |
C |
|
|
|
E |
4 |
H |
F |
|
|
|
|
|
Answer the following questions based on the table.
Answers to the questions
Students may be asked to complete the following paragraph using the appropriate terms. Ionic bond is made by the electrostatic attraction force between (1) ________ and (2) ________ ions that are formed from (3) ________ elements and (4) _________ elements by losing and gaining electrons respectively. Ionic bond is also called (5) ________ bond. In ionic compounds all the anions are surrounded by (6) ________ and cations are surrounded by (7) ________; but the cations and anions depends up on the number of (8) ________ gained and lost. Ionic compounds can be represented by (9) ________ formula in which the dotes are used instead of (10) _______ of the atoms.