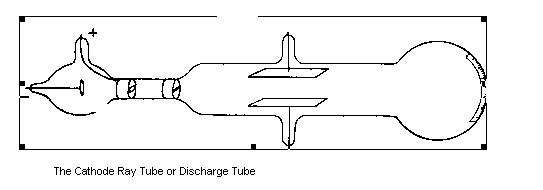
When a high potential difference was applied across the electrodes a stream of particles passed between them
|
Back Unit 1.1
|
Home Cover Page
|
Top Unit 1.2
|
Next Unit 1.3
|
Unit One: Structure of the Atom
Materials
Revision of the modern atomic theory Let the student’s state postulates of modern atomic theory focusing on its basic difference from Dalton’s atomic theory. Make sure that slow learners have understood that atoms are divisible. This can be done by asking them to state and interpret the postulates. You may write the important point of the postulates they mention on the black board. Students should be invited to amend the ideas forwarded by their friends.
Evaluation:
Ask students to predict how scientists came to know the presence of particles in an atom. Appreciate any idea they may forward. This may give you some information about students’ preconception regarding atoms and their particles.
Then give a brief summary of the famous experiment carried out by Thomson using the discharge tube diagram. This could include the following points:

When a high potential difference was applied across the electrodes a stream of particles passed between them
Make students into groups depending on your class size. You may give the following group activities. The activities may be written on a piece of paper in copies to distribute to groups or on a chart and posted on the wall for all students to see or on the chalk board.
Discuss the following and give reasons
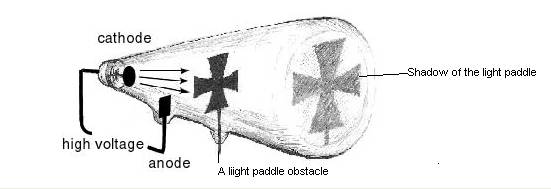
Generalize as in the following table
No |
Observation |
Findings |
1 |
Rotation of light paddle |
|
2 |
Shadow casted on the screen |
|
3 |
Attraction of the ray to the positive terminal |
|
4 |
Observation of the same result for different gases |
|
Let the group representatives come in front to the chalk board and write their findings. The class should comment on the responses of each group.
Ask students to state all the observations and explain their findings
Give the students opportunity to ask questions.
Let other students respond to the posed questions.
You may comment on the answer if necessary
Ask some students randomly to give summary of the day’s lesson. Make sure that students are able to explain that:
Students should discuss how, from this evidence, Thomson was able to state that: